How to Find the Density of a Fluid if You Know the Pressure

Density Measurement
Definition, Equations, Influences, Instruments, Density vs Relative Density and More than
Density measurements check the purity and concentration of a sample and gives insight into its composition. The measurement of density is crucial in dissimilar industries to ensure quality for both raw materials and finished goods.
For example, the density of ultrapure water at xx.00 °C is known to exist 0.998203 yard/cmiii: any deviation from this value ± tolerances would imply that the water sample contains impurities.
On this page, y'all will gain essential knowledge almost density measurement.
Jump to one of the following sections to explore and learn more virtually density of liquids.
- Density Explained
- How to Measure the Density of Liquids
- Factors That Impact Density Decision
- Temperature
- Air Bubbling or Impurities
- Air Pressure of a Fluid
- Viscosity
- Relative Density, Specific Gravity, True Density: What It Is and Its Differences
- How to Ensure Good Density Results with a Digital Density Meter: The 5-Steps to Success
- Temperature
- FAQ
i. Density Explained
What Is Density?
Density is a physical parameter that provides information on the mass of a sample or body divided past its volume: in other words, how tightly a substance'southward molecules are packed together in space. The density is usually represented by the Greek letter rho "ρ". The Latin alphabetic character "d" is as well commonly used.
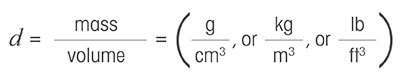

Have you ever wondered why some oils stay on the body of water's surface when there is an oil spill?
The difference in densities makes some substances ascent higher up others, in the image yous tin come across examples of typical density values demonstrated.
A - Lamp Oil (0.80 yard/cm3 )
B - Rubbing Alcohol (0.87 g/cmiii)
C - Vegetable Oil (0.91 grand/cm3 )
D - Water (0.99 m/cm3 )
E - Dawn Dish Lather (1.33 g/cmiii)
F - Honey (1.36 chiliad/ccm3 )
two. How to Measure the Density of Liquids
The table below summarizes each measurement method, including their strengths and weaknesses.
Hydrometers
 |
Measuring principle
- Glass trunk inserted into sample
- Glass body floats at a certain level due to the buoyancy and mass of the hydrometer, dependent on sample density
- Level of equilibration shows the density on the calibrated calibration
Strengths
- Elementary, cheap
- Used for a quick cheque of an judge density value
Weaknesses
- User-dependent results
- Takes a long time to equilibrate the temperature
- Small measuring range (typically takes 20 hydrometers to cover a wide range)
- Large sample volume required (140 mL to 600 mL)
- Hard to clean
- Non suitable for GLP
- Breakable
- Sample must be removed from sample container and poured into the hydrometer
Pycnometers
 |
Measuring principle
- Drinking glass beaker of defined volume
- Weighed without sample (M1), then with a sample (M2)
- Density calculated based on the post-obit formula:
Density = (M2 − M1)/Flask Volume
Strengths
- Inexpensive
- Directly related to the definition of density (mass divided by book): platonic for academia / education
Weaknesses
- User-dependent results
- Pycnometers are calibrated for a certain temperature, east.m. xx °C, and then measurements are only valid at that temperature! The sample must be equilibrated to the scale temperature.
- Density must be calculated
- Typical sample volume required is 25 mL
- High level of user training required to ensure accurate, trustworthy measurements
- Sample must exist removed from sample container and added to pycnometer
Digital Density Meters
 |
Measuring principle
- Oscillating U-tube
- A hollow drinking glass tube vibrates at a certain frequency. This frequency changes when the tube is filled with the sample: the college the mass of the sample, the lower the frequency measurement is and converted into density. A built-in Peltier thermostat controls the temperature precisely of the benchtops musical instrument (no water bathroom required)
Strengths
- Easy to use
- Small sample volume
- Automatic measurement means results are operator contained
- Built-in temperature compensation
- Offers storage of upwards to 1100 results and the possibility to connect to PC software for data management
- Sample tin be measured directly from the sample container
Weaknesses
- More expensive in comparison to hydrometers or pycnometers
Using a Pycnometer to Determine Density?
Sentry this video to check if you are following the correct steps for a successful density measurement with a pycnometer. Furthermore, we compare density measurement with a pycnometer vs a digital density meter.
3. Factors That Affect Density Determination
a) Temperature
Did y'all know that a temperature change of 0.1 °C can have an impact of 0.0001 1000/cm3 on the measured density?
The temperature influences the space necessary to fit atoms in a molecule. The vibration increases with higher temperature, moving the atoms further apart and therefore reducing the density value.
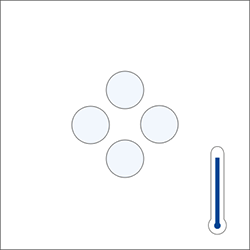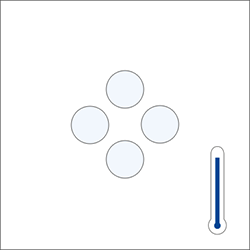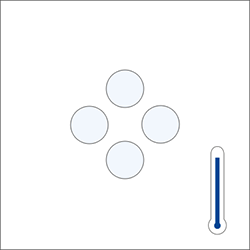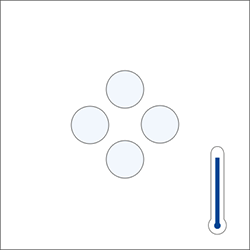 |
Molecule at a given temperature
(slight movements)
 |
Same molecule when temperature increases
(moving further autonomously)
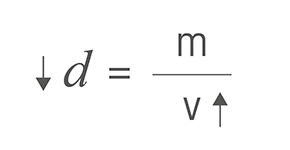
Therefore, the higher the temperature, the higher the volume and the lower the density. If the temperature decreases, the volume becomes lower and the density becomes greater. Only, the mass of the substance does not change.
The only exception to this rule is liquid water, which reaches its density pinnacle at 3.98 ºC, above this betoken the volume of water increases and information technology becomes less dense. The opposite applies when water is cooled.
Notation: The relation among temperature, book and density is not a linear part and it depends on the specific heat chapters, heat of vaporization and other factors of each substance.
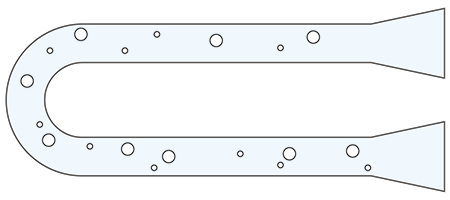
b) Air Bubbles or Impurities
A simple air bubble present in a liquid measured can crusade a massive difference to its density and the same applies for impurities.
For instance, a glass pycnometer relies on the mass for the calculation of the density value. If in that location is an air bubble present or a contamination east.grand. due to improper cleaning, the mass measured and displayed past the balance will be incorrect. This leads to an incorrect density value.

- The same applies for a digital density meter, which relies on the U-tube measuring principle for the determination of density.
- Nonetheless, modern digital density meters feature an automatic BubbleCheck™ to support the user during the density measurement process.
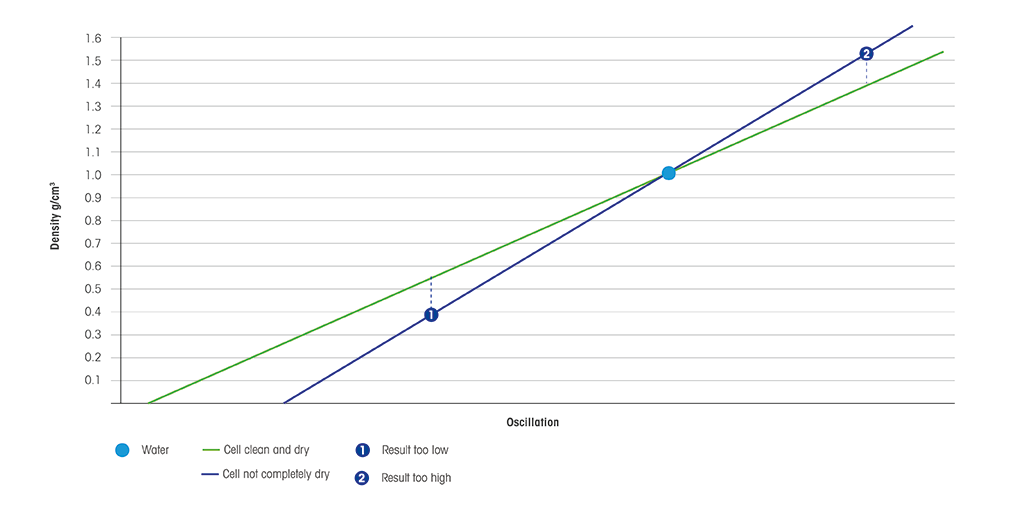
- Moreover, to avert cross contagion due to improper cleaning, users can measure the density of air. If the effect of the measurements is inside the limits, this means that the measuring prison cell has been properly cleaned.
c) Air Pressure of a Fluid
If you lot are in Mexico City at 3.930 meters in a higher place the sea level, the local air pressure will exist lower than in Rio de Janeiro, which is at sea level or 0 meters. This ways the air pressure is straight related to the altitude.
As gases and liquids are fluids, the following information must be considered:
- Gas (e.g. air), is a compressible fluid, which changes its volume at unlike pressures.
- Liquids (e.g. water), are considered incompressible fluid, so their book is constant at different pressures. If extremely high air pressure would be applied, liquids could become compressible, but this is not the case for analytical density measurement purposes.

In a density measurement using a transmission instrument (e.g. pycnometer), the air pressure level value is not taken into consideration.
Mod digital density meters feature a built in barometer (pressure sensor) to mensurate the local air pressure, which automatically sets the reference air density value. This value is important for ii reasons:
- During instrument adjustment, h2o and air value must be measured. The pressure has an effect on the air value and therefore on all measurements after this.
Learn more than near instrument adjustment at present! Download our gratuitous density measurement guide - During daily operations, the cleanliness of a measuring cell must be conducted to ensure expert results by fugitive cross contamination. The air density value measured is compared to the reference air value prepare. If the result is within a certain tolerance, the musical instrument jail cell is make clean.

d) Viscosity
If a substance is viscous, is it denser than a less viscous liquid?
Viscosity describes the resistance of a liquid in flowing, informally described as the "thickness" of a fluid, and in principle, it has no direct relationship with density.
Nonetheless, the viscosity may influence the density determination depending on the method used.
Measurements using:
- Pycnometer: Non influenced, but slow sampling and cleaning, and slow temperature stabilization when using water bath.
- Hydrometers: Non influenced, simply slow sampling, value reading and cleaning.
- Digital density meter: Influenced, because sample dampens the oscillation frequency of the vibrating U-tube.
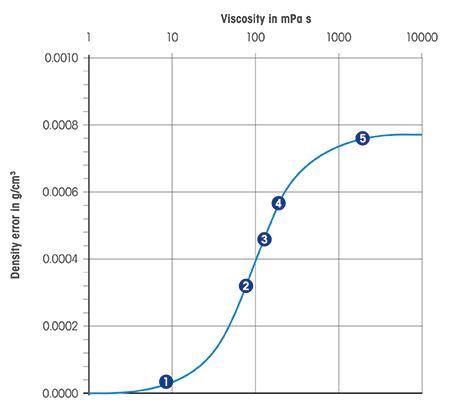
- Every bit seen in the chart, equally the viscosity increases, the density mistake too increases.
- However, modern digital density meters automatically correct errors due to viscosity, ensuring maximum accuracy and fast measurements.
- Aqueous solutions, lab (organic) solvents
- Sulfuric solutions
- Motor oil
- Kitchen detergent
- Syrup
east) Relative Density, Specific Gravity, True Density: What It Is and Its Differences

Relative density definition
Relative density is the ratio between absolute densities of two substances, where the divisor is considered the reference substance. If this substance is not specified, it is causeless to exist water at 3.98ºC and therefore has a density of 0.999972 g/cm3 or 999.972 kg/miii. As per the equation, the relative density has no units.

Specific gravity definition
Specific gravity (SG) is the ratio between the density of a substance and the density of water. If the temperature is not specified, it is assumed to be h2o at 3.98ºC and therefore has a density of 0.999972 g/cmthree or 999.972 kg/mthree.
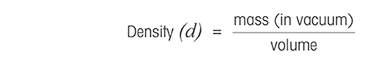
True density or absolute density definition
Truthful density is the ratio between the mass and volume of a substance at a given pressure and temperature, respective to its weight in a vacuum. This is the concept also used in density measurement by digital density meters.
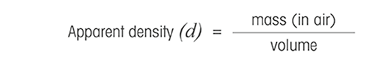
Apparent density definition
Apparent density is a property of partitioned solids, such as powders and granules, and is oft used in the mining, food and chemic industries. Past definition, credible density is the ratio between mass and volume, but it corresponds to weight in air.
 |
| Mass in vacuum = True density |
To illustrate the difference between true density and apparent density, we have a pycnometer placed onto a balance. When information technology is filled with a liquid, it weighs less in air than in a vacuum due to the buoyancy event of air.
Many official density tables are still based on apparent density. Digital density meters deliver results in unlike units and concentrations, cheque out their specifications.
 |
| Mass in air = Credible density |
f) How to Ensure Adept Density Results with a Digital Density Meter: The 5 Steps to Success
Ensuring accurate density measurement results requires taking directly activeness to avoid inaccuracy throughout the workflow process. Below yous volition discover important tips for the success of your density determination.
Step 1 – Make up one's mind Your Sample Type / Sample Preparation
Before you begin your measurement, it is important to sympathise the necessary precautions to take depending on your sample type.
Tips:
Mucilaginous
- Ensure no air bubbles are present upon introduction into the measuring cell
- Shear forces can cause inaccurate results, so brand sure your instrument has a viscosity correction
Aggressive / toxic
- If full-bodied acids or bases (e.thou. H2SO4, HCl, NaOH) are measured, minimize contact and evaporation by using an automated system with an autosampler
Volatile
- Samples containing dissolved organic gas (due east.g. butane), show a tendency to degas, then they should be cooled downwardly to avoid chimera generation.
Sample with dissolved gases
- Carbonated samples, for case soft drinks, must be degassed prior to measurement. To do and then, stir them for several minutes until bubbles stops.
- For samples with dissolved air, an ultra-sonic bath can be used, or the sample tin be boiled for several minutes.
- Remove COii or dissolved gas with a combination of air flow and stirrer with an automated density system
Non-homogeneous or with suspensions
- Avoid solid material settle or a concentration slope germination
- Stir the sample well earlier taking a sample. Brand sure that no air bubbles are introduced during stirring.
- If you lot can't completely homogenize the sample, repeat the measurement several times and calculate the mean value.
Step 2 – Test Your Density Meter
Regularly verify the measurement performance of your arrangement by measuring a sample of accurately known density (eastward.g. ultrapure water or a liquid standard). This procedure is called test, scale or bank check. Later completing the check or test, the measured density is compared to the known nominal value of the sample.

Additionally, it is skilful practice to bank check that the measuring cell is fully clean and dry out between the analyses of different substances. It is recommended that on a daily basis y'all complete a test of your density meter with ultrapure water (0.998203 yard/cm3 at 20.00 °C).
Some common questions are:
Which tolerance should be applied for a test with deionized water?
And for organic standards?
What to do to if the test fails?

Step 3 – Preclude Sampling Errors
Did yous know that a bubble of 2 mm diameter can crusade an error of 0.0008 grand/cm3 on your result?
If you are measuring with a syringe:
- We recommend "overfilling" the cell past at least an extra 5 cm at the outlet
- Fill up the measuring cell at a constant dull speed and with a laminar flow to ensure complete wetting of the jail cell walls (no trapped bubbles forth the walls)
When filling automatically with a pump or autosampler:
- Adjust the sampling speed according to the sample viscosity
Prevent bubbles - After filling, cheque if the cell is bubble-free
- Yous tin easily identify bubbling with a live video of the measuring cell and automatic Bubblecheck™ with a digital density meter

Step 4 – Measure out and Document Your Results
Nonetheless converting your results manually?
Looking up in or interpolating from a tabular array is error-prone and time-consuming. In addition, hand-written results conduct the take a chance of transcription errors. Depending on the environment, at that place is no guarantee that this kind of documentation fulfills regulatory requirements.
The most user-friendly solution is an automatic conversion using congenital-in tables (eastward.g. alcohol, Brix, temperature compensation according to API), or also user defined, which prevents reading or calculation errors and saves fourth dimension. A digital density meter allows the apply of built-in conversion tables to prove the result directly in the desired unit of measurement.
Step 5 – Clean Your Density Meter
Inappropriate cleaning is the most mutual source of erroneous results!
Make sure that the measuring prison cell does non contain any remainder from previously measured samples or rinsing solutions
To foreclose this, the cell should be cleaned with suitable rinsing solutions and dried – preferably after each measurement
Some full general recommendations for the rinsing solutions:
Cleaning solution 1
- Must completely deliquesce your sample (e.g. Deconex 12% or h2o)
Cleaning solution 2
- Must dissolve the cleaning solution 1, and evaporate quickly (e.g. Acetone or ethanol)
Finally, make sure to dry the prison cell completely with dry out air. In many cases, there is no need to use two solvents when using METTLER TOLEDO Density Meters. Thank you to the powerful DryPro pump, you lot can salve time and solvent consumption.
Of import: Adjusting a density meter
Frequent adjustments of the musical instrument does not necessarily guarantee authentic results. Any adjustment results in changes beingness fabricated to the instrument's internal settings. If an aligning is performed incorrectly, this will then pb to incorrect measurements.
Therefore, nosotros recommend the adjustment of the density meter only if the test has failed several times repeatedly
FAQ
- What Is the Difference between Density and Mass?
- Why Tin Density Exist Used to Identify a Sample?
- How Is the Density of Solutions Measured?
- Is Density Directly Proportional to Pressure?
- What Are Some Typical Applications of Density Measurement?
- What Is the Density of Air?
- What Is the Density of Water?
- What Is the Influence of Viscosity on Digital Density Measurement?
What Is the Deviation between Density and Mass?
Mass is a measure of how much matter there is within an object or liquid while density expresses how much mass there is per a certain corporeality of book.
For instance, 10 kg of steel and x kg of feathers take the same mass, but different volumes therefore they take dissimilar densities.
Why Can Density Exist Used to Identify a Sample?
Density can hands be used to identify a pure sample considering each chemical element has a unique density. After a measurement, the density of the sample in question can be looked upward to see what it corresponds to.
How Is the Density of Solutions Measured?
Let's take a solution of ethanol in h2o equally an example.
Every bit shown earlier, at 20 °C pure water has a density of d = 0.9982 thou/cmthree, and pure ethanol has a density of d = 0.7892 g/cm3 at 20 °C. A solution of ethanol/water will have a density value which depends on the concentration of the solution.
Is Density Directly Proportional to Pressure?
Density is directly proportional to the local air pressure merely indirectly proportional to temperature. At a constant temperature, when pressure increases density increases. Learn more near the human relationship between density of liquids and force per unit area here.
What Are Some Typical Applications of Density Measurement?

Some applications of density measurement includes the determination of alcohol concentration in spirits, control of fermentation process in vino and beer product, Brix (carbohydrate content) measurement of intermediate and final products in food and beverages products. Density and other concentration every bit API gravity in heavy oils, alkane and lubricants in the Petrochemical segment. Density (specific gravity) in battery acid in the automobile industry, also as other solvents, acids and bases in the chemical industry. Lastly, there are many applications in the pharmaceutical industry, such as the density measurement of specific gravity in cosmetics, personal care products, and many more. Click on the links to a higher place to read our detailed applications or admission our expertise library to find the right awarding note according to your sample.
What Is the Density of Air?
The density of air is 0.00120 grand/cm3 at xx°C and under atmospheric force per unit area of 101.325 kPa (i.east. at sea level). This atmospheric pressure level changes with the conditions conditions (lower force per unit area when rainy or snowy) and with the altitude (lower pressure at high distance than at sea level). At an superlative of 440 m above sea level, for example, the atmospheric pressure (yearly hateful) is 96.12 kPa merely and the hateful air density becomes 0.00114 g/cm3 at 20°C.
Learn more
What Is the Density of H2o?
The density of water is 0.99820 g/cmiii at 20°. The density of water changes with the temperature. It increases from 0°C to 4°C (where it is nearly 1) and and so decreases from 4°C to higher temperatures.
What Is the Influence of Viscosity on Digital Density Measurement?
Sample viscosity has an touch on the density measured with a digital density meter. The higher shear force which occurs between the fluid and the tube wall results in slowing downwardly the oscillation frequency, thus showing a higher density value. Modern digital density meters therefore have a built-in viscosity correction, which compensates for this effect to show correct results.


Products

Excellence Density Meters
Adult for a wide range of applications, Excellence density meters allow for workflow automation and multiparameter systems. Measure almost any sample with high reliability and...

Standard EasyPlus Density Meters
With a remarkably elementary user interface, EasyPlus Density Meters allow anyone to obtain accurate results in the lab or most the production line.

Portable Density Meter
Densito portable density meter: lightweight and like shooting fish in a barrel to use! Measure out different types of samples inside seconds.
bohlerereepliefor.blogspot.com
Source: https://www.mt.com/sg/en/home/applications/Application_Browse_Laboratory_Analytics/Density/density-measurement.html


0 Response to "How to Find the Density of a Fluid if You Know the Pressure"
Post a Comment